How to Spot the Early Symptoms of Melanoma
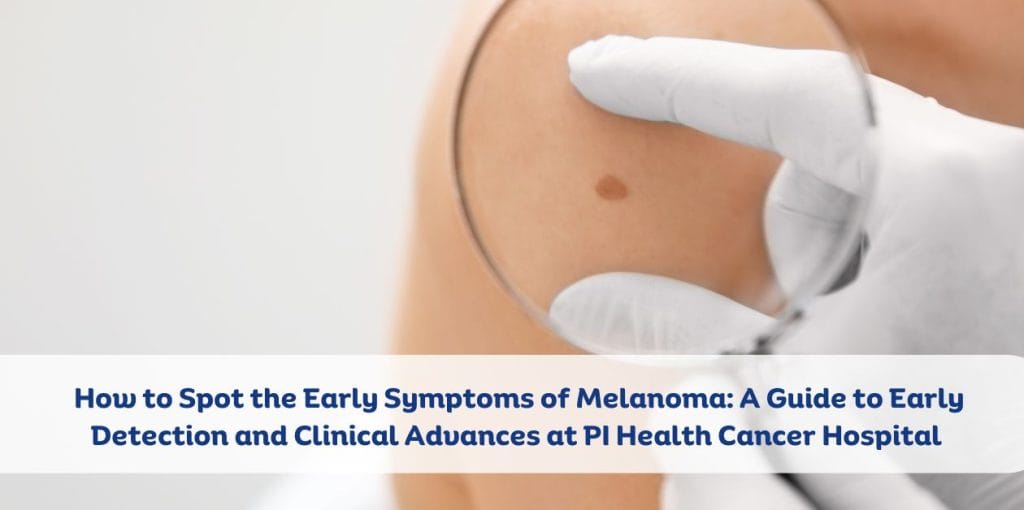
Home >> How to Spot the Early Symptoms of Melanoma: A Guide to Early Detection and Clinical Advances at PI Health Cancer Hospital Melanoma is a dangerous form of skin cancer that develops in the pigment-producing cells (melanocytes) of the skin. While it accounts for only a small percentage of all skin cancers, it is responsible for most skin cancer-related deaths. Early detection of melanoma is crucial for effective treatment and improved survival rates. In this blog, we will discuss how to spot the early symptoms of melanoma and explore groundbreaking clinical trials at PI Health Cancer Hospital that are advancing cancer detection and treatment methods. Understanding Melanoma: What You Need to Know Melanoma can develop in any part of the body, but it is mostly found on the skin. It is often caused by sun exposure, though it can also develop in areas that are not typically exposed to the sun. The cancer can spread quickly to other parts of the body if not caught early, making early detection critical. Common Skin Cancer Symptoms to Watch Out For Knowing how to identify the early symptoms of melanoma can be lifesaving. The key to detecting melanoma early lies in recognizing changes to the skin. Here are the most common signs to look for: Changes in Existing Moles: If you have a mole or birthmark that changes in shape, color, size, or texture, it could be a sign of melanoma. These changes may occur over weeks or months. New Growths on the Skin: Melanoma may also appear as a new, unusual growth on the skin, often asymmetrical, with uneven borders and varying colors. Itchiness, Bleeding, or Pain: If a mole or new growth becomes itchy, painful, or starts to bleed, it’s important to get it checked by a healthcare provider immediately. Color Changes: Melanomas often have multiple colors, such as black, brown, red, or even blue. This is different from benign moles, which tend to have a uniform color. Unusual Shape or Border: Unlike normal moles, which are typically round or oval with smooth borders, melanomas may have irregular, jagged, or scalloped borders. Changes in Size: A mole or growth that increases in size, especially if it is larger than the size of a pencil eraser, should be closely monitored. The Role of Sun Exposure in Melanoma While anyone can develop melanoma, exposure to ultraviolet (UV) radiation from the sun or tanning beds is one of the leading risk factors for developing skin cancer. UV rays damage the DNA in skin cells, leading to mutations that can result in cancer. It’s important to note that melanoma can occur in areas that are not typically sun-exposed, so even those with limited sun exposure should remain vigilant about skin changes. Additionally, people with fair skin, a history of sunburns, or a family history of skin cancer are at a higher risk. How PI Health Cancer Hospital is Advancing Melanoma Detection and Treatment PI Health Cancer Hospital is at the forefront of melanoma research, conducting groundbreaking clinical trials that focus on innovative methods for early detection and treatment. These clinical trials are exploring new ways to identify melanoma at its earliest stages, allowing for more effective treatments and better outcomes for patients. Clinical Trials Focused on Early Cancer Detection One of the most exciting advancements in melanoma research at PI Health Cancer Hospital is the development of non-invasive screening technologies. These include innovative imaging techniques and molecular markers that can detect melanoma before visible symptoms appear. By identifying melanoma early, doctors can begin treatment while the cancer is still confined to the skin, significantly improving the patient’s prognosis. Immunotherapy and Targeted Treatments In addition to early detection, PI Health Cancer Hospital is also pioneering clinical trials that explore the use of immunotherapy and targeted treatments for melanoma. Immunotherapy works by boosting the body’s natural defenses to recognize and fight cancer cells, while targeted therapies aim at specific genes or proteins that drive cancer growth. These treatments offer promising results for patients with advanced melanoma, and clinical trials are helping to refine their effectiveness. Personalized Cancer Care Another key focus of PI Health Cancer Hospital’s clinical trials is personalized cancer care. By analyzing each patient’s unique genetic makeup, doctors can tailor treatments to the individual’s specific needs. This personalized approach has shown tremendous success in improving treatment outcomes and minimizing side effects. How to Protect Yourself from Melanoma While early detection is essential, prevention is equally important. Here are some tips to help reduce your risk of developing melanoma: Use Sunscreen: Always apply broad-spectrum sunscreen with SPF 30 or higher, even on cloudy days. Reapply every two hours, or more frequently if swimming or sweating. Avoid Tanning Beds: Tanning beds expose the skin to elevated levels of UV radiation, increasing the risk of melanoma. Wear Protective Clothing: When spending time outdoors, wear protective clothing, wide-brimmed hats, and sunglasses to reduce sun exposure. Seek Shade: Avoid prolonged exposure to the sun, especially during peak hours (10 a.m. to 4 p.m.). Regular Skin Checks: Perform regular self-exams of your skin to check for any new or changing moles. Schedule annual checkups with a dermatologist for professional skin evaluations. Conclusion Melanoma is a serious skin cancer, but with early detection, it is highly treatable. Understanding the symptoms of melanoma and getting regular checkups is essential for protecting your health. Thanks to groundbreaking clinical trials at PI Health Cancer Hospital, early detection methods are becoming more advanced, leading to more effective treatments. By staying vigilant, protecting your skin, and seeking professional help when needed, you can take steps toward preventing and fighting melanoma. If you notice any unusual changes in your skin or have concerns about your risk for melanoma, do not hesitate to contact a healthcare provider. Initial action can make all the difference. FAQS 1. What are the first signs of melanoma? The first signs of melanoma include changes in the shape, color, or size of existing moles, or the appearance of new, unusual growths on
How to Spot the Early Symptoms of Melanoma

Home >> How to Spot the Early Symptoms of Melanoma: A Guide to Early Detection and Clinical Advances at PI Health Cancer Hospital Melanoma is a dangerous form of skin cancer that develops in the pigment-producing cells (melanocytes) of the skin. While it accounts for only a small percentage of all skin cancers, it is responsible for most skin cancer-related deaths. Early detection of melanoma is crucial for effective treatment and improved survival rates. In this blog, we will discuss how to spot the early symptoms of melanoma and explore groundbreaking clinical trials at PI Health Cancer Hospital that are advancing cancer detection and treatment methods. Understanding Melanoma: What You Need to Know Melanoma can develop in any part of the body, but it is mostly found on the skin. It is often caused by sun exposure, though it can also develop in areas that are not typically exposed to the sun. The cancer can spread quickly to other parts of the body if not caught early, making early detection critical. Common Skin Cancer Symptoms to Watch Out For Knowing how to identify the early symptoms of melanoma can be lifesaving. The key to detecting melanoma early lies in recognizing changes to the skin. Here are the most common signs to look for: Changes in Existing Moles: If you have a mole or birthmark that changes in shape, color, size, or texture, it could be a sign of melanoma. These changes may occur over weeks or months. New Growths on the Skin: Melanoma may also appear as a new, unusual growth on the skin, often asymmetrical, with uneven borders and varying colors. Itchiness, Bleeding, or Pain: If a mole or new growth becomes itchy, painful, or starts to bleed, it’s important to get it checked by a healthcare provider immediately. Color Changes: Melanomas often have multiple colors, such as black, brown, red, or even blue. This is different from benign moles, which tend to have a uniform color. Unusual Shape or Border: Unlike normal moles, which are typically round or oval with smooth borders, melanomas may have irregular, jagged, or scalloped borders. Changes in Size: A mole or growth that increases in size, especially if it is larger than the size of a pencil eraser, should be closely monitored. The Role of Sun Exposure in Melanoma While anyone can develop melanoma, exposure to ultraviolet (UV) radiation from the sun or tanning beds is one of the leading risk factors for developing skin cancer. UV rays damage the DNA in skin cells, leading to mutations that can result in cancer. It’s important to note that melanoma can occur in areas that are not typically sun-exposed, so even those with limited sun exposure should remain vigilant about skin changes. Additionally, people with fair skin, a history of sunburns, or a family history of skin cancer are at a higher risk. How PI Health Cancer Hospital is Advancing Melanoma Detection and Treatment PI Health Cancer Hospital is at the forefront of melanoma research, conducting groundbreaking clinical trials that focus on innovative methods for early detection and treatment. These clinical trials are exploring new ways to identify melanoma at its earliest stages, allowing for more effective treatments and better outcomes for patients. Clinical Trials Focused on Early Cancer Detection One of the most exciting advancements in melanoma research at PI Health Cancer Hospital is the development of non-invasive screening technologies. These include innovative imaging techniques and molecular markers that can detect melanoma before visible symptoms appear. By identifying melanoma early, doctors can begin treatment while the cancer is still confined to the skin, significantly improving the patient’s prognosis. Immunotherapy and Targeted Treatments In addition to early detection, PI Health Cancer Hospital is also pioneering clinical trials that explore the use of immunotherapy and targeted treatments for melanoma. Immunotherapy works by boosting the body’s natural defenses to recognize and fight cancer cells, while targeted therapies aim at specific genes or proteins that drive cancer growth. These treatments offer promising results for patients with advanced melanoma, and clinical trials are helping to refine their effectiveness. Personalized Cancer Care Another key focus of PI Health Cancer Hospital’s clinical trials is personalized cancer care. By analyzing each patient’s unique genetic makeup, doctors can tailor treatments to the individual’s specific needs. This personalized approach has shown tremendous success in improving treatment outcomes and minimizing side effects. How to Protect Yourself from Melanoma While early detection is essential, prevention is equally important. Here are some tips to help reduce your risk of developing melanoma: Use Sunscreen: Always apply broad-spectrum sunscreen with SPF 30 or higher, even on cloudy days. Reapply every two hours, or more frequently if swimming or sweating. Avoid Tanning Beds: Tanning beds expose the skin to elevated levels of UV radiation, increasing the risk of melanoma. Wear Protective Clothing: When spending time outdoors, wear protective clothing, wide-brimmed hats, and sunglasses to reduce sun exposure. Seek Shade: Avoid prolonged exposure to the sun, especially during peak hours (10 a.m. to 4 p.m.). Regular Skin Checks: Perform regular self-exams of your skin to check for any new or changing moles. Schedule annual checkups with a dermatologist for professional skin evaluations. Conclusion Melanoma is a serious skin cancer, but with early detection, it is highly treatable. Understanding the symptoms of melanoma and getting regular checkups is essential for protecting your health. Thanks to groundbreaking clinical trials at PI Health Cancer Hospital, early detection methods are becoming more advanced, leading to more effective treatments. By staying vigilant, protecting your skin, and seeking professional help when needed, you can take steps toward preventing and fighting melanoma. If you notice any unusual changes in your skin or have concerns about your risk for melanoma, do not hesitate to contact a healthcare provider. Initial action can make all the difference. FAQS 1. What are the first signs of melanoma? The first signs of melanoma include changes in the shape, color, or size of existing moles, or the appearance of new, unusual growths on
Lung Cancer Staging and Its Impact on Treatment Options at PI Health Cancer Hospital
Home >> Lung Cancer Staging and Its Impact on Treatment Options at PI Health Cancer Hospital Lung cancer remains one of the leading causes of cancer-related deaths worldwide, but advancements in early detection, diagnosis, and treatment are helping to improve patient outcomes. One of the most critical steps in the diagnosis of lung cancer is staging—the process of determining the size and spread of the cancer. The stage of lung cancer significantly influences treatment options, prognosis, and overall management strategies. At PI Health Cancer Hospital, we specialize in providing precise staging of lung cancer to ensure personalized, effective treatment plans. In this blog, we will explore the importance of lung cancer staging, how it impacts treatment options, and how PI Health Cancer Hospital offers comprehensive care to patients at every stage of their diagnosis. What is Lung Cancer Staging? Lung cancer staging refers to the process by which doctors determine the extent to which lung cancer has spread within the body. This stage is essential because it helps oncologists create the most effective treatment strategy. The stage of cancer is determined based on several factors, including the tumor’s size, its location in the lungs, whether it has spread to nearby lymph nodes, and if it has metastasized (spread) to other parts of the body. Lung cancer staging is typically done through a combination of imaging tests, biopsies, and physical exams. The most common imaging tests used for staging lung cancer are: CT scans MRI scans PET scans Chest X-rays These tests help doctors identify the size of the tumor and check for any spread to lymph nodes or distant organs. In some cases, a biopsy is necessary to determine the cancer’s type and cell characteristics. The Four Stages of Lung Cancer Lung cancer is classified into two main types: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). These two types are staged differently, and understanding these stages is crucial for developing an effective treatment plan. 1. Non-Small Cell Lung Cancer (NSCLC) Stages: NSCLC is the most common lung cancer type, staged using the TNM staging system, which considers the size of the tumor (T), lymph node involvement (N), and whether the cancer has spread to other parts of the body (M). The stages for NSCLC are as follows: Stage 0 (In situ): The cancer is confined to the surface layer of the lung and has not spread beyond the lung. Stage I: The tumor is small and localized within the lung, with no spread to lymph nodes. Stage II: The tumor may be larger or has spread to nearby lymph nodes. Stage III: The cancer has spread to more distant lymph nodes or other structures near the lungs, such as the chest wall or diaphragm. Stage IV: The cancer has metastasized to other organs such as the liver, bones, or brain. 2. Small Cell Lung Cancer (SCLC) Stages: SCLC is a more aggressive form of lung cancer, and it is typically staged as either limited stage or extensive stage: Limited Stage: The cancer is confined to one side of the chest and can be treated with radiation and chemotherapy. Extensive Stage: The cancer has spread to both sides of the chest or to other parts of the body, often requiring more intensive treatment options like chemotherapy and immunotherapy. How Lung Cancer Staging Affects Treatment Options The stage of lung cancer plays a pivotal role in determining the most appropriate treatment plan. At PI Health Cancer Hospital, our oncologists use advanced diagnostic tools to accurately stage the cancer, ensuring that each patient receives the most effective treatment tailored to their specific condition. Below, we outline the treatment options typically associated with each stage of lung cancer. Stage 0: In this earliest stage, the cancer is localized and has not spread. Treatment for Stage 0 lung cancer often involves surgery to remove the tumor, and in some cases, a minimally invasive procedure like bronchoscope biopsy or wedge resection may be used. Radiation therapy is also an option for certain patients. The prognosis at this stage is typically very favorable, and many patients can be cured with appropriate treatment. Stage I: For Stage I non-small cell lung cancer, surgery is usually the first line of treatment. This may involve a lobectomy, in which a portion of the lung is removed, or a pneumonectomy in more advanced cases. If surgery is not possible due to the tumor’s location, radiation therapy or a combination of radiation and chemotherapy may be used. Stage II: Stage II lung cancer may also be treated with surgery, but it is often followed by adjuvant chemotherapy to reduce the risk of recurrence. Radiation therapy may be recommended if surgery is not an option, and chemotherapy may be combined with it for better efficacy. For patients who are not candidates for surgery, more advanced treatments like immunotherapy may be considered. Stage III: At Stage III, lung cancer may be more challenging to treat, but there are still options available. Treatment may include a combination of chemotherapy, radiation therapy, and in some cases, immunotherapy, or targeted therapies. In some cases, surgery may be an option if the tumor is respectable. Clinical trials exploring newer treatments may also be an option for patients in this stage. Stage IV: In the advanced stage of lung cancer, the goal of treatment shifts to palliative care to manage symptoms and improve the quality of life. For patients with Stage IV NSCLC, treatments like chemotherapy, targeted therapies, immunotherapy, and palliative radiation therapy are commonly used. In many cases, treatments are aimed at slowing the progression of the disease and controlling symptoms rather than curing it. For extensive-stage SCLC, chemotherapy is the standard treatment, often combined with immunotherapy to improve outcomes. Radiation therapy may also be used to manage symptoms. Lung Cancer Treatment at PI Health Cancer Hospital At PI Health Cancer Hospital, we provide the latest in lung cancer treatment and care. Our team of specialists works closely with patients to ensure that
Lung Cancer Diagnosis: Tests and Procedures You Should Know About

Home >> Lung Cancer Diagnosis: Tests and Procedures You Should Know About Lung cancer is one of the leading causes of cancer-related deaths worldwide, and early diagnosis plays a crucial role in improving treatment outcomes. With advancements in medical research and technology, lung cancer diagnosis has become more accurate and efficient. In this blog, we will explore the essential tests and procedures used for lung cancer diagnosis, with a focus on the groundbreaking clinical trials being conducted at PI Health Cancer Hospital. These innovations are reshaping the way lung cancer is diagnosed and treated, offering new hope for patients. Understanding Lung Cancer Diagnosis Lung cancer is typically diagnosed through a combination of imaging tests, biopsies, and other diagnostic procedures. These tests help doctors determine the presence, size, and location of the tumor, and whether the cancer has spread to other parts of the body. Early detection is key to improving survival rates, as lung cancer is often diagnosed at an advanced stage. This makes regular screening and awareness of symptoms crucial. Common Symptoms of Lung Cancer Before we dive into the diagnostic procedures, it’s important to recognize some common symptoms of lung cancer. While symptoms can vary, here are the most common signs: Persistent cough or changes in a chronic cough Shortness of breath Chest pain or discomfort Wheezing Blood in sputum (coughed-up mucus) Unexplained weight loss or fatigue If you experience any of these symptoms, it’s essential to consult a healthcare provider, who can recommend the appropriate tests to diagnose or rule out lung cancer. Key Tests and Procedures for Lung Cancer Diagnosis Chest X-Ray A chest X-ray is often the first imaging test used to check for lung cancer. It provides a basic image of the lungs and can reveal the presence of abnormal growths or tumors. Although a chest X-ray can indicate potential lung cancer, it is not always definitive. Further tests are typically required for confirmation. 2. CT scan (Computed Tomography) A CT scan provides detailed, cross-sectional images of the lungs and is more effective at detecting lung cancer than a chest X-ray. It can help doctors locate tumors, assess their size, and determine whether cancer has spread to nearby tissues. CT scans are also used to guide biopsies and other procedures. 3. MRI (Magnetic Resonance Imaging) While CT scans are primarily used to examine the lungs, an MRI may be recommended if doctors need to assess whether cancer has spread to the brain or spinal cord. MRI uses magnetic fields to create detailed images of soft tissues and organs. 4. PET Scan (Positron Emission Tomography) A PET scan is often used to determine the stage of lung cancer by highlighting areas of increased metabolic activity, which can indicate the presence of cancer cells. This test is particularly useful for detecting cancer that has spread beyond the lungs. 5. Bronchoscopy In cases where a tumor is in the airways, a bronchoscopy may be performed. During this procedure, a thin, flexible tube (bronchoscope) is inserted through the nose or mouth and into the lungs. The bronchoscope allows the doctor to visualize the airways, collect tissue samples (biopsy), and clear any blockages. 6. Biopsy A biopsy is the most definitive way to diagnose lung cancer. During a biopsy, a small sample of tissue from the suspected tumor is removed and examined under a microscope for cancerous cells. There are several ways to perform a biopsy, including bronchoscopy, CT-guided needle biopsy, or surgery, depending on the location of the tumor. 7. Sputum Cytology Sputum cytology involves examining a sample of sputum (mucus or phlegm) under a microscope to look for cancer cells. While this test is not as commonly used as other diagnostic procedures, it can be helpful for diagnosing certain types of lung cancer, especially in patients who have a persistent cough or are at elevated risk. Groundbreaking Clinical Trials at PI Health Cancer Hospital PI Health Cancer Hospital is a leader in cancer research, conducting numerous clinical trials aimed at improving lung cancer diagnosis, treatment, and outcomes. These trials are focused on enhancing current diagnostic methods and exploring new techniques for earlier detection. Liquid Biopsy for Early Detection One of the most promising areas of research at PI Health Cancer Hospital is the development of liquid biopsy technology. Liquid biopsies analyze blood samples for genetic material released by cancer cells. This non-invasive test can potentially detect lung cancer in its earliest stages, even before visible tumors form. Liquid biopsies are also being tested to monitor treatment response and detect any recurrence of cancer, making it an essential tool in personalized cancer care. Artificial Intelligence (AI) in Cancer Detection Another exciting development at PI Health Cancer Hospital involves artificial intelligence (AI) to enhance diagnostic accuracy. AI algorithms are being trained to analyze medical images such as CT scans and X-rays, identifying subtle patterns that might be missed by the human eye. These technologies are expected to revolutionize lung cancer screening, making it faster, more accurate, and accessible to a broader population. Targeted Lung Cancer Screening Lung cancer screening is recommended for individuals at elevated risk, such as smokers or those with a family history of lung cancer. PI Health Cancer Hospital is conducting clinical trials to refine screening techniques and identify the most effective methods for early detection. Low-dose CT scans, currently the standard for high-risk individuals, are being tested alongside other advanced screening methods to determine their effectiveness. How Early Detection Improves Lung Cancer Prognosis Lung cancer, especially in its initial stages, is often asymptomatic, which is why regular screenings are essential for high-risk individuals. Early-stage lung cancer is more likely to be localized to the lungs and may be surgically removed or treated with targeted therapies. The prognosis for patients diagnosed at this stage is significantly better than for those diagnosed at later stages when cancer has spread to other parts of the body. In fact, when lung cancer is caught early, the five-year survival rate can be as high as 56%, compared to only
Early Symptoms of Liver Cancer That You Shouldn’t Ignore

Home >> Early Symptoms of Liver Cancer That You Shouldn’t Ignore Liver cancer, also known as hepatic cancer, is one of the most dangerous types of cancer, often diagnosed at an advanced stage due to its subtle early symptoms. Identifying the early signs of liver disease is crucial for timely treatment, as early detection significantly improves outcomes. At PI Health Cancer Hospital, we focus on educating patients and the public about liver cancer symptoms and the importance of routine screenings and checkups. In this blog, we will discuss the key early symptoms of liver cancer that you should never ignore and explain how they are linked to the development of hepatic cancer. We will also highlight the importance of seeking medical attention when you experience these signs. 1. Unexplained Weight Loss One of the first signs of liver cancer is unexplained weight loss. This occurs when the body’s metabolism is altered due to the presence of cancer cells in the liver. If you are losing weight without making any lifestyle changes, it is essential to monitor your condition. Why it Happens: As the liver becomes overwhelmed by cancer cells, it cannot function properly. This disruption leads to the body’s difficulty in metabolizing food and nutrients, causing weight loss. 2. Abdominal Pain and Swelling Abdominal discomfort or pain, particularly in the upper right side where the liver is located, is another common early sign of liver cancer. Swelling in the abdomen or a feeling of fullness after eating a small amount can also be an indication of liver disease. Why it Happens: As the tumor grows, it places pressure on surrounding tissues, causing pain, and swelling. Fluid buildup (ascites) in the abdomen is another cause of swelling, which can be a sign of advanced liver disease. 3. Fatigue and Weakness Liver cancer often causes overwhelming fatigue, making it difficult to perform daily activities. Fatigue may also be accompanied by a feeling of weakness and a lack of energy, even after rest. Why it Happens: The liver’s compromised ability to filter toxins and produce important substances like albumin can lead to fatigue. Additionally, the body’s fight against cancer cells consumes a lot of energy. 4. Loss of Appetite and Nausea A sudden loss of appetite, especially when coupled with persistent nausea, could signal the development of liver cancer. People with liver disease often feel nauseous or have difficulty eating even small portions of food. Why it Happens: Tumors in the liver can press on the stomach or digestive system, affecting the ability to eat. Moreover, the liver’s failure to process bile properly can result in nausea. 5. Yellowing of the Skin or Eyes (Jaundice) Jaundice, a yellowing of the skin or the whites of the eyes, is a common sign of hepatic cancer. This condition occurs when the liver is no longer able to process bilirubin properly, leading to its buildup in the bloodstream. Why it Happens: As liver function deteriorates due to cancer, the organ cannot remove excess bilirubin, a byproduct of red blood cell breakdown, causing yellowish discoloration. 6. Dark Urine and Pale Stools Changes in the color of urine and stools can indicate liver dysfunction. Dark, tea-colored urine and pale, clay-colored stools are signs that you should not ignore. Why it Happens: Dark urine is a result of bilirubin in the bloodstream, while pale stools indicate a lack of bile being properly processed by the liver, both of which are signs of liver disease and cancer. 7. Itchy Skin Itching, or pruritus, is a symptom that can arise from liver disease or liver cancer. The buildup of bile acids in the bloodstream causes the skin to become irritated and itchy, particularly on the hands and feet. Why it Happens: When the liver is not functioning properly, bile acids accumulate in the bloodstream, leading to skin irritation and itching. This symptom is often more noticeable at night. When to See a doctor If you are experiencing any combination of the above symptoms, it is essential to seek medical attention as soon as possible. Early detection of liver cancer is crucial, as it significantly increases the chances of effective treatment and can even save lives. At PI Health Cancer Hospital, our expert oncologists and liver specialists are equipped with advanced diagnostic tools to detect and treat liver cancer at its earliest stages. Conclusion Recognizing the early symptoms of liver cancer is vital for early intervention and successful treatment. If you experience any of the signs discussed in this blog, do not hesitate to consult a healthcare professional at PI Health Cancer Hospital. Early detection plays a key role in increasing survival rates and improving the quality of life for patients with hepatic cancer. Regular screenings and awareness are the first steps toward preventing this life-threatening disease. FAQS 1. What are the main causes of liver cancer? Liver cancer is primarily caused by chronic liver diseases such as hepatitis B or C, cirrhosis, and fatty liver disease. Excessive alcohol consumption, obesity, and certain genetic conditions can also increase the risk of developing liver cancer. 2. How is liver cancer diagnosed? Liver cancer is diagnosed through a combination of imaging tests (such as CT scans, MRIs, and ultrasounds), blood tests (including liver function tests), and a biopsy, where a sample of liver tissue is examined under a microscope. 3. Is liver cancer preventable? While liver cancer itself may not always be preventable, certain lifestyle changes can reduce your risk, such as avoiding excessive alcohol use, maintaining a healthy weight, and getting vaccinated against hepatitis B. Regular liver checkups are also recommended for people with a higher risk of developing liver disease. 4. What are the stages of liver cancer? Liver cancer is typically classified into four stages, ranging from initial stages (localized tumors) to advanced stages (tumors that have spread to other parts of the body). Treatment options and prognosis depend on the cancer’s stage at the time of diagnosis. 5. Can liver cancer be treated? Yes, liver cancer can be treated,
The Role of Hematology in Cancer Diagnosis and Treatment at PI Health Cancer Hospital
Home >> The Role of Hematology in Cancer Diagnosis and Treatment at PI Health Cancer Hospital Hematology is a specialized branch of medicine that focuses on the study of blood, blood-forming organs, and blood disorders. In the context of cancer care, hematology plays a crucial role in diagnosing and treating blood cancers such as leukemia, lymphoma, and myeloma. At PI Health Cancer Hospital, our hematology department is at the forefront of providing advanced, personalized care for patients suffering from blood cancers. In this blog post, we will delve into the critical role of hematology in cancer diagnosis and treatment, focusing on blood cancers and their management. Understanding Hematology and Cancer Hematology is essential in understanding the behavior of blood cancers and ensuring timely and accurate diagnosis. Blood cancers, which include leukemia, lymphoma, and myeloma, affect the blood cells, bone marrow, lymphatic system, and spleen. These cancers disrupt the normal functioning of blood cells, impairing the body’s ability to fight infections, transport oxygen, and clot properly. At PI Health Cancer Hospital, we use state-of-the-art diagnostic tools and treatments to address blood cancers. Our hematology team is highly experienced in diagnosing and treating these complex diseases using a range of modern methods, including molecular diagnostics, chemotherapy, stem cell therapy, and targeted therapies. The Importance of Early Diagnosis in Blood Cancer Treatment The earlier blood cancer is detected, the better the chances of successful treatment. Haematologists specialize in identifying subtle changes in blood cells, often before symptoms become evident to the patient. Early detection can lead to more effective treatments and better patient outcomes. Common diagnostic tests used in hematology include: Complete Blood Count (CBC): This is often the first step in detecting abnormal blood cells, including leukemia cells. Bone Marrow Biopsy: This test helps confirm the diagnosis of blood cancers and provides detailed information about the cancer type. Flow Cytometry and Cytogenetic Tests: These tests identify specific cancer markers and chromosomal abnormalities, essential for personalized treatment plans. At PI Health Cancer Hospital, we combine these diagnostic tools with the latest advancements in hematology to ensure precise and early diagnosis, improving the quality of life for our patients. Key Blood Cancers Diagnosed and Treated at PI Health Cancer Hospital Leukemia: Leukemia is a cancer of the blood and bone marrow, characterized by an overproduction of abnormal white blood cells. There are different types of leukemia, including acute and chronic forms, which can affect both children and adults. Hematologists at PI Health Cancer Hospital specialize in identifying the subtype of leukemia and tailoring treatment plans accordingly. Treatments may include chemotherapy, targeted therapy, and stem cell transplants. Lymphoma: Lymphoma affects the lymphatic system, which is an essential part of the immune system. There are two main types of lymphoma: Hodgkin lymphoma and non-Hodgkin lymphoma. Our team uses advanced diagnostic techniques such as PET scans and biopsies to determine the specific type and stage of lymphoma. Treatment options include chemotherapy, radiation therapy, immunotherapy, and bone marrow transplants. Myeloma: Multiple myeloma is a cancer that affects plasma cells in the bone marrow, leading to abnormal production of antibodies. PI Health Cancer Hospital offers a comprehensive treatment approach for myeloma, including chemotherapy, stem cell transplants, and novel therapies like immunomodulatory drugs and proteasome inhibitors. Advanced Hematology Treatment Options at PI Health Cancer Hospital At PI Health Cancer Hospital, our hematology team is committed to providing the most advanced and effective treatments for blood cancers. These treatments are designed to target cancerous cells while minimizing damage to healthy cells, ensuring the best possible outcomes for our patients. Chemotherapy and Targeted Therapy:Chemotherapy remains a cornerstone in cancer treatment. At PI Health Cancer Hospital, we use chemotherapy in conjunction with targeted therapy, which focuses on specific molecular targets to halt cancer cell growth. This combination improves the effectiveness of the treatment. Stem Cell and Bone Marrow Transplants:For patients with blood cancers like leukemia or myeloma, stem cells or bone marrow transplants can be lifesaving. These procedures replace damaged bone marrow with healthy stem cells, enabling the body to produce healthy blood cells again. Immunotherapy:Immunotherapy is an emerging field in cancer treatment that harnesses the body’s immune system to fight cancer. Our team at PI Health Cancer Hospital is using immunotherapy to treat blood cancers with promising results, providing hope to patients who have not responded to traditional treatments. Personalized Cancer Care at PI Health Cancer Hospital – Hematology in Cancer Each patient is unique, and at PI Health Cancer Hospital, we believe that cancer treatment should be personalized to meet the specific needs of every individual. Our hematology specialists work closely with other oncology experts to create comprehensive treatment plans that address the entire patient – not just the cancer. By considering factors such as the patient’s overall health, cancer subtype, and genetic makeup, we can offer more precise treatments that have a higher chance of success. We also emphasize supportive care to manage symptoms, improve quality of life, and promote healing throughout the treatment process. Conclusion The role of hematology in cancer diagnosis and treatment is invaluable, especially when it comes to blood cancers such as leukemia, lymphoma, and myeloma. At PI Health Cancer Hospital, we provide the highest level of care, ensuring that each patient receives the most advanced and personalized treatment options available. Our hematology specialists work tirelessly to provide accurate diagnoses, implement targeted therapies, and support our patients throughout their cancer journey. If you or a loved one is facing blood cancer, PI Health Cancer Hospital is here to help. Contact us today to learn more about how we can support you through your diagnosis and treatment. FAQS 1. What is the role of hematology in cancer diagnosis? Hematology helps diagnose blood cancers through tests like CBC, bone marrow biopsies, and molecular diagnostics. Early diagnosis allows for targeted treatment, improving patient outcomes. 2. What are the main types of blood cancers? The main types of blood cancers include leukemia, lymphoma, and myeloma, each of which affects different blood components and requires specialized treatment. 3. How are blood cancers
What You Need to Know About Gastric Cancer Treatment Options

Home >> What You Need to Know About Gastric Cancer Treatment Options Gastric cancer, also known as stomach cancer, is a serious and often difficult-to-diagnose disease that affects the stomach lining. While the survival rates for gastric cancer have historically been low, recent advancements in cancer therapies and cutting-edge clinical trials have significantly improved outcomes for patients. At PI Health Cancer Hospital, groundbreaking research is being conducted to enhance both the diagnosis and treatment of this complex condition. This blog will delve into gastric cancer treatment options, the latest developments in stomach cancer treatment, and how clinical trials at PI Health Cancer Hospital are paving the way for new and improved therapies for those affected by this disease. What is Gastric Cancer? Gastric cancer begins in the stomach, usually in the cells of the stomach lining. It is one of the most common types of cancer worldwide, though its prevalence has decreased in some regions due to better dietary habits and early screening practices. However, stomach cancer is often diagnosed at later stages, making it more challenging to treat effectively. There are several risk factors for gastric cancer, including infection with Helicobacter pylori bacteria, smoking, family history, and a diet high in salty or processed foods. Symptoms may include persistent stomach pain, bloating, nausea, difficulty swallowing, and unexplained weight loss. Since these symptoms can overlap with other conditions, early detection is crucial. Stomach Cancer Treatment Options The treatment for gastric cancer depends on various factors, including the stage of the cancer, its location, the patient’s overall health, and the presence of metastasis (spread of cancer). The treatment strategy often involves a combination of therapies to improve outcomes. 1. Surgery For localized gastric cancer, surgery is the primary treatment option. The goal is to remove the tumor and any affected tissue. Common surgical procedures include: Gastrectomy: Removal of part or all of the stomach. Lymph node removal: In some cases, nearby lymph nodes may be removed to reduce the risk of cancer spreading. While surgery is effective in early-stage cancer, it may not always be an option for advanced-stage gastric cancer, particularly if the cancer has spread to other organs. 2. Chemotherapy Chemotherapy is often used in combination with surgery to eliminate cancer cells that may have spread beyond the tumor site. It involves the use of powerful drugs that target and kill rapidly dividing cells, including cancer cells. Chemotherapy may be administered before or after surgery, or in some cases, as the primary treatment if surgery is not possible. Recent advancements in chemotherapy for gastric cancer have led to the development of more targeted drugs that aim to minimize damage to healthy cells while focusing on cancerous cells. 3. Radiation Therapy Radiation therapy uses high-energy rays to destroy cancer cells. It is generally used in combination with surgery or chemotherapy to shrink tumors before surgery or to target remaining cancer cells after surgery. Radiation may also be used to treat gastric cancer that has spread to other areas, such as nearby lymph nodes or the liver. Recent studies have explored more precise radiation techniques, such as intensity-modulated radiation therapy (IMRT), which can focus on the tumor while minimizing exposure to surrounding healthy tissues. 4. Targeted Therapy Targeted therapy involves drugs that target specific molecules involved in the growth and spread of cancer. These therapies are designed to attack cancer cells more precisely than traditional chemotherapy, often leading to fewer side effects. For gastric cancer, targeted therapies are used to block specific proteins that promote cancer cell growth. At PI Health Cancer Hospital, clinical trials are exploring several promising targeted therapies that could improve survival rates and quality of life for gastric cancer patients. 5. Immunotherapy Immunotherapy is a relatively new approach in cancer treatment. It works by stimulating the patient’s immune system to recognize and destroy cancer cells. Several immunotherapy drugs have shown promise in treating gastric cancer, particularly for advanced or metastatic cases. For example, checkpoint inhibitors are a class of immunotherapy drugs that block certain proteins from preventing immune cells from attacking cancer cells. Ongoing clinical trials at PI Health Cancer Hospital are testing new immunotherapy combinations for gastric cancer patients. Groundbreaking Clinical Trials at PI Health Cancer Hospital PI Health Cancer Hospital is a leader in cancer research and gastric cancer treatments. The hospital’s clinical trials focus on investigating new drugs, treatment combinations, and advanced diagnostic methods to improve outcomes for gastric cancer patients. Some of the key areas of research include: Personalized Medicine: By analyzing the genetic makeup of cancer cells, researchers at PI Health Cancer Hospital are developing personalized treatment plans tailored to individual patients, maximizing the effectiveness of therapy. Novel Targeted Therapies: Clinical trials are exploring drugs that target specific mutations in gastric cancer cells, potentially offering more effective treatment options with fewer side effects. Immunotherapy Combinations: Ongoing trials are testing the potential of combining immunotherapy with other cancer treatments, such as chemotherapy or radiation, to enhance their effectiveness. Liquid Biopsy: Researchers are investigating the use of liquid biopsies—blood tests that detect genetic mutations in gastric cancer—to enable earlier diagnosis and more accurate monitoring of the disease. By participating in these trials, PI Health Cancer Hospital is playing a vital role in improving gastric cancer treatment options and outcomes. Conclusion Gastric cancer is a challenging disease, but recent advances in cancer therapies are offering hope for better outcomes. By utilizing a combination of surgery, chemotherapy, radiation, and innovative treatments like targeted therapy and immunotherapy, gastric cancer patients now have more options than ever. At PI Health Cancer Hospital, cutting-edge clinical trials are helping to push the boundaries of treatment, providing patients with access to the latest breakthroughs in stomach cancer treatment. Early detection, personalized care, and advancements in gastric cancer treatment are crucial in improving survival rates and quality of life for those affected by this disease. FAQS 1. What are the first signs of gastric cancer? Early signs of gastric cancer may include persistent stomach pain, bloating, indigestion, unexplained weight loss, and
What Does the Future Hold for Biosimilar Nivolumab in Treating NSCLC?

Home >> What Does the Future Hold for Biosimilar Nivolumab in Treating NSCLC? Lung cancer, particularly non-small cell lung cancer (NSCLC), remains one of the leading causes of cancer-related deaths globally. Despite advances in treatment, many patients still face limited options and prohibitive costs for effective therapies. This is where biosimilar nivolumab comes into play, offering a promising alternative to the original monoclonal antibody treatment for NSCLC. As the treatment landscape continues to evolve, biosimilars like nivolumab have the potential to transform the way NSCLC is managed. Groundbreaking clinical trials, such as those conducted at PI Health Cancer Hospital, are shedding light on the future of biosimilar nivolumab in the fight against NSCLC. What Is Biosimilar Nivolumab? Before diving into its prospects, it is essential to understand what biosimilar nivolumab is. Nivolumab, originally developed by Bristol-Myers Squibb, is a checkpoint inhibitor designed to enhance the immune system’s ability to fight cancer cells. It works by blocking the PD-1 protein, which cancer cells use to evade immune detection. While nivolumab has shown substantial promise in treating cancers like NSCLC, it remains a high-cost therapy. Biosimilars are biologic medications that are identical to an already approved reference product (in this case, nivolumab). They are produced once the patent for the original drug expires. Biosimilar nivolumab provides the same therapeutic benefits as its reference product but at a potentially lower cost, making it a more accessible option for NSCLC patients. The Role of Clinical Trials in Advancing Biosimilar Nivolumab At PI Health Cancer Hospital, innovative clinical trials are exploring the efficacy and safety of biosimilar nivolumab in treating NSCLC. These trials have become a cornerstone in evaluating the potential of biosimilars in oncology. The findings from these trials are particularly exciting as they are expected to confirm whether biosimilar nivolumab can deliver the same clinical outcomes as the reference drug, at a fraction of the cost. Recent trials at PI Health have involved comparing biosimilar nivolumab with the reference nivolumab in treating both advanced and early-stage NSCLC. Results from early-stage trials suggest that biosimilar nivolumab matches the reference product in terms of progression-free survival, overall survival, and overall safety profiles. This breakthrough has significant implications for patients’ access to life-saving treatments, especially in regions with limited healthcare resources. Prospects for Biosimilar Nivolumab in NSCLC Treatment The future of biosimilar nivolumab in NSCLC treatment looks promising, as it could provide numerous benefits to both patients and healthcare systems. Here are some key factors to consider Improved Patient Access The excessive cost of cancer treatments is a major barrier for many patients. By introducing biosimilars into the market, more patients will have access to effective treatments that were previously out of reach. As clinical trials continue to demonstrate its efficacy, biosimilar nivolumab will be seen as a viable first-line treatment for NSCLC. Cost-Effectiveness One of the most significant advantages of biosimilar nivolumab is its potential for cost-effectiveness. The lower cost of biosimilars can reduce the financial burden on both patients and healthcare systems. This is especially critical in cancer treatment, where the costs of care can quickly escalate. Biosimilar nivolumab could lead to savings that could be reinvested into other areas of cancer care, such as early detection and patient support services. FDA Approval and Global Adoption The FDA approval of biosimilar nivolumab is a critical step for its widespread use. Once approved, it will pave the way for other regulatory bodies worldwide to grant approval, making the treatment more accessible to patients across various countries. Given the promising results from ongoing clinical trials, FDA approval could be granted soon, accelerating its entry into the market. Improved Patient Outcomes Clinical trials at PI Health Cancer Hospital have shown that biosimilar nivolumab offers comparable survival benefits to the reference nivolumab. This means that patients with advanced NSCLC may experience the same positive outcomes as those treated with the original drug, but at a much lower cost. This could lead to an improved quality of life for patients, as more people can access life-saving therapies. Conclusion The future of biosimilar nivolumab in the treatment of NSCLC looks incredibly promising, with groundbreaking clinical trials, such as those at PI Health Cancer Hospital, paving the way for improved patient outcomes. With its potential for cost-effectiveness, better patient access, and eventual FDA approval, biosimilar nivolumab could redefine the landscape of NSCLC treatment. As clinical trials continue to show its safety and efficacy, it is likely that this innovative treatment will become a cornerstone in the fight against lung cancer, helping both patients and healthcare systems worldwide. Read This : Managing Hypertension: Breakthrough Treatments and Lifestyle Changes in 2024 Cervical Cancer: Causes, Symptoms, Screening, Prevention and Treatment FAQS 1. Can a healthy diet really prevent colon cancer? Yes, a diet high in fiber, fruits, vegetables, and whole grains can reduce your risk of colon cancer. Limiting red and processed meats, while emphasizing plant-based foods, helps protect against the development of colorectal cancer. 2. At what age should I start getting screened for colon cancer? The general recommendation is to begin screening at age 45 for individuals at average risk. If you have a family history or genetic predisposition, talk to your healthcare provider about starting screenings earlier. 3. How does exercise help prevent colon cancer? Exercise helps maintain a healthy weight, regulates digestion, and promotes healthy bowel movements—all of which contribute to reducing the risk of colon cancer. 4. What are the early signs of colon cancer? Early signs of colon cancer can include persistent abdominal pain, changes in bowel habits (like diarrhea or constipation), blood in the stool, unexplained weight loss, and fatigue. If you notice any of these symptoms, seek medical advice. Dr. A. Venugopal Clinical Director & HOD Medical Oncology Senior Consultant Medical Oncologist & Hemato-Oncologist View Profile About Author Dr. A. Venugopal MD (General Medicine), DM (Medical Oncology), MRCP – SCE Medical Oncology (UK), ECMO (Switzerland). Dr A. Venugopal is One of the best medical oncologist and Hemato Oncologist in hyderabad, currently serving as
What Are the Early Symptoms of Colon Cancer?
Home >> What Are the Early Symptoms of Colon Cancer? Colon cancer, also known as colorectal cancer, is one of the most common types of cancer worldwide. It develops in the colon or rectum and often begins with the growth of abnormal cells, leading to symptoms that may appear gradually over time. Early detection of colon cancer is crucial for successful treatment, but many people may not experience obvious symptoms in the initial stages. Understanding the early symptoms of colon cancer is key to early diagnosis and improved outcomes. In this blog, we will explore the early symptoms of colon cancer, explain how they relate to gastrointestinal cancer, and provide important insights on what to look for. Additionally, we will answer common questions about colon cancer symptoms to help you understand this potentially life-threatening disease better. What is Colon Cancer? Colon cancer, or colorectal cancer, refers to the cancer that begins in the large intestine (colon) or rectum. This type of cancer can start as small, non-cancerous growths called polyps, which over time may develop into cancer. While colon cancer can occur without symptoms, early warning signs can help detect the disease before it progresses to later stages. Early Symptoms of Colon Cancer Colon cancer often presents with vague symptoms in the early stages, which can easily be mistaken for other less serious gastrointestinal issues. However, it is important to be aware of any persistent changes to your health, especially if they involve the gastrointestinal system. Early detection is key to improving survival rates, so if you experience any of the following symptoms, it’s essential to consult a healthcare professional for further evaluation. Changes in Bowel Habits One of the most common signs of colon cancer is a noticeable change in bowel habits. This may include persistent diarrhea or constipation that lasts for more than a few days, or a change in the consistency of your stool. Colon cancer can affect how the colon functions, causing an increase or decrease in bowel movements or altering stool appearance, such as narrow or thin stools. Abdominal Discomfort or Cramping Abdominal discomfort, cramps, or bloating are often associated with colon cancer. As the tumor grows, it can cause a blockage in the colon, which may lead to persistent discomfort or a feeling of fullness. Some individuals report a bloated or gassy sensation that doesn’t go away. Blood in Stool or Rectal Bleeding The presence of blood in the stool, either bright red or dark, is one of the most concerning symptoms of colon cancer. This can occur when the tumor bleeds into the gastrointestinal tract. If you notice blood on the toilet paper, in your stool, or in the toilet bowl, it’s crucial to seek medical advice, as this could indicate colon cancer or other gastrointestinal issues, such as hemorrhoids. Unexplained Weight Loss Unintentional weight loss, without any changes to your diet or exercise routine, may be an early sign of colon cancer. Weight loss occurs when the cancer interferes with the body’s ability to absorb nutrients, or it can be due to the increased metabolic demands of the growing tumor. This symptom, along with other gastrointestinal cancer signs, should be taken seriously. Fatigue and Weakness Persistent fatigue or a general sense of weakness is another common early symptom of colon cancer. Colon cancer can cause anemia (a deficiency in red blood cells) due to internal bleeding or malnutrition, leading to symptoms like tiredness, weakness, and dizziness. If you’re feeling unusually fatigued without a clear cause, it’s important to consult a healthcare provider. Feeling of Incomplete Bowel Movements Colon cancer can cause a feeling that your bowel movements are incomplete, even after going to the bathroom. This sensation is often linked to the tumor’s obstruction of the colon, which prevents it from fully emptying. If you regularly feel like you have not fully evacuated your bowels, this could be a symptom of colon cancer. Iron Deficiency Anemia Colon cancer can lead to chronic blood loss, which in turn can cause iron deficiency anemia. This condition can make you feel unusually tired, weak, or short of breath. Iron deficiency anemia is often detected through a blood test, and it can sometimes be the first clue that doctors will investigate when symptoms of colon cancer are present. How Colon Cancer Develops Colon cancer typically begins as non-cancerous growths called polyps on the inner lining of the colon. Over time, these polyps may develop into cancerous tumors. In some cases, colon cancer can grow without causing symptoms, which is why it is often detected in later stages when it has already spread to other areas. Regular screenings, such as colonoscopies, are vital in detecting precancerous polyps before they turn into cancer. If you are over 50 or have a family history of colorectal cancer, regular screenings are essential for early detection. Risk Factors for Colon Cancer While the exact cause of colon cancer is not always known, several risk factors can increase your likelihood of developing the disease: Age: Colon cancer is more common in people over 50, although younger individuals can also be diagnosed. Family History: A family history of colon cancer or polyps increases your risk. Diet: A diet high in red meat, processed foods, and low in fiber may increase the risk. Personal Health History: Individuals with a history of inflammatory bowel diseases like Crohn’s disease or ulcerative colitis are at higher risk. Lifestyle Factors: Smoking, excessive alcohol consumption, and lack of physical activity can also contribute to an increased risk of colon cancer. Conclusion Colon cancer is a serious condition that often develops silently, but early detection through awareness of its symptoms can make a significant difference in treatment outcomes. By recognizing the early symptoms of colon cancer—such as changes in bowel habits, blood in the stool, unexplained weight loss, and fatigue—you can take proactive steps to seek medical advice and undergo screening. If you or someone you know is experiencing any of these symptoms, it’s important not to ignore them. Consult with a healthcare professional to determine
How to Prevent Colon Cancer: Tips for a Healthy Lifestyle

Home >> How to Prevent Colon Cancer: Tips for a Healthy Lifestyle Colon cancer, also known as colorectal cancer, is one of the most common types of cancer worldwide. However, with the right lifestyle choices, many cases of colon cancer can be prevented. Colon cancer prevention starts with understanding the risk factors, making healthy dietary choices, staying active, and getting regular screenings. At PI Health Cancer Hospital, we emphasize the importance of colon cancer prevention and the role of a healthy lifestyle in reducing the risk of developing this disease. In this blog, we’ll provide actionable tips on how to lower your risk of colorectal cancer through diet, exercise, and early cancer screening, helping you live a healthier, cancer-free life. Understanding Colon Cancer and Its Risk Factors Colon cancer begins in the large intestine (colon) or rectum and often develops from polyps—small growths on the inner lining of the colon. Over time, some of these polyps can become cancerous. Risk Factors for Colon Cancer While some risk factors for colon cancer, such as age and family history, are out of your control, many others are related to lifestyle choices. Here are some common risk factors: Age: People over the age of 50 are at a higher risk. Family History: Having a first-degree relative with colon cancer increases your risk. Diet and Cancer: A diet high in red or processed meats and low in fiber has been linked to a higher risk of colon cancer. Lack of Physical Activity: A sedentary lifestyle contributes to a higher risk. Obesity: Being overweight or obese can increase the likelihood of developing colon cancer. Now that we understand the risk factors, let’s dive into practical ways to reduce those risks. Top Tips for Preventing Colon Cancer – How to Prevent Colon Cancer Maintain a Healthy Diet Your diet plays a crucial role in colon cancer prevention. A balanced, nutritious diet can reduce your risk of colorectal cancer significantly. Focus on: Fiber-rich foods: Include plenty of fruits, vegetables, legumes, and whole grains. Fiber helps keep the digestive system healthy and promotes regular bowel movements, reducing the chances of colon cancer. Limit red and processed meats: Studies have shown that a diet high in red meat (such as beef, pork, and lamb) and processed meats (like bacon and sausage) increases your risk. Instead, opt for lean proteins like poultry, fish, and plant-based proteins. Increase antioxidants: Foods rich in antioxidants, such as berries, nuts, and leafy greens, may help protect against cellular damage and reduce inflammation in the body. Stay hydrated: Drinking plenty of water aids digestion and helps prevent constipation, a known risk factor for colorectal cancer. Exercise Regularly Physical activity is essential for maintaining a healthy weight and preventing obesity, which is a significant risk factor for colon cancer. Regular exercise also helps regulate bowel movements and improves digestion. Aim for 150 minutes of moderate aerobic activity, like walking or swimming, each week, along with strength training exercises twice a week. Consistency is key. Engaging in physical activity regularly can help lower your risk of colorectal cancer and improve your overall health. Achieve and Maintain a Healthy Weight Being overweight or obese increases the risk of colon cancer, particularly after the age of 50. Achieving a healthy weight through diet and exercise can reduce this risk. If you’re unsure about your ideal weight, consult your healthcare provider for personalized advice. Monitor your waist circumference: A larger waistline is linked to an increased risk of colorectal cancer, so maintaining a healthy body composition is important. Balanced meals: Instead of crash diets, focus on long-term, sustainable eating habits that prioritize whole, unprocessed foods. Avoid Tobacco and Limit Alcohol Intake Both smoking and excessive alcohol consumption are linked to an increased risk of colorectal cancer. By quitting smoking and limiting alcohol intake, you can significantly reduce your cancer risk. Quit smoking: Smoking is a known carcinogen, and avoiding tobacco is one of the best ways to prevent cancer. Moderate alcohol consumption: Limit alcohol intake to no more than one drink per day for women and two drinks per day for men. Get Regular Cancer Screenings Early detection is key in preventing colorectal cancer. Regular cancer screenings, such as colonoscopies, can help detect precancerous polyps and treat them before they develop into cancer. Screening Recommendations: People at average risk should begin colorectal cancer screening at age 45. If you have a family history of colon cancer or other risk factors, you may need to start screening earlier or undergo more frequent screenings. Discuss with Your Doctor: Your healthcare provider will guide you on the best screening options for you, which may include a colonoscopy, stool tests, or other non-invasive screenings. Know Your Family History and Genetic Risk Genetic factors play a significant role in the development of colorectal cancer. If you have a family history of colon cancer, it’s crucial to inform your doctor. They may recommend earlier and more frequent screenings or genetic counseling to better understand your risk. Conclusion Preventing colon cancer is possible with the right lifestyle choices. By focusing on a healthy diet, regular physical activity, and early cancer screenings, you can significantly reduce your risk of developing colorectal cancer. At PI Health Cancer Hospital, we are dedicated to providing education and support for our patients, offering the latest in cancer screening and prevention. If you’re due for a screening or need personalized guidance on colon cancer prevention, don’t hesitate to contact us today. Read This : Comprehensive Guide to Dengue Fever: Symptoms, Treatment, and Prevention – Pi Health Cancer Hospital Chemotherapy FAQS 1. Can a healthy diet really prevent colon cancer? Yes, a diet high in fiber, fruits, vegetables, and whole grains can reduce your risk of colon cancer. Limiting red and processed meats, while emphasizing plant-based foods, helps protect against the development of colorectal cancer. 2. At what age should I start getting screened for colon cancer? The general recommendation is to begin screening at age 45 for individuals at average risk. If
